How To Draw Lewis Structures .doc .pdf
9.3: Drawing Lewis Structures
- Folio ID
- 170032
Learning Objectives
- To describe Lewis Structures for molecules and polyatomic ions with ane central atom.
Introduction to Lewis structures
A Lewis structure is a way to testify how atoms share electrons when they form a molecule. Lewis structures bear witness all of the valence electrons in an cantlet or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis construction of an atom, write the symbol of the cantlet and depict dots around it to represent the valence electrons.
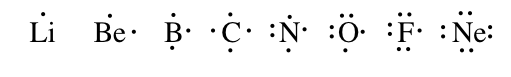
Note that hydrogen is frequently shown in both group 1A and group 7A, only information technology has one valence electron – never seven. Also, helium is shown in grouping 8A, only it only has two valence electrons.
Representing a Covalent Bond Using Lewis Structures
Nonmetals tin can form a chemical bond by sharing two electrons. Each atom contributes ane electron to the bond. For example, two hydrogen atoms can form a bond, producing a molecule of Htwo. Using Lewis structures, we can represent this as follows:

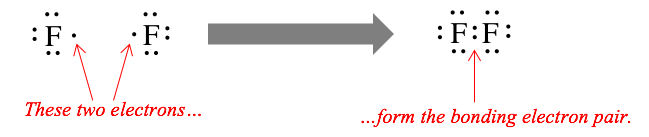
Two fluorine atoms can form a molecule of F2 in the same manner. Note that each cantlet must contribute one electron to the bond.
Atoms tin can form more than one bond. In a water molecule, an oxygen atom forms 2 bonds, one to each hydrogen atom.

Chemists commonly represent a bail using a line instead of two dots. The structures of Htwo, Ftwo, and HtwoO would ordinarily exist fatigued as follows:
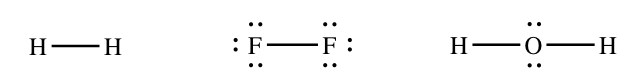
Merely the bonding electrons are shown using lines. Nonbonding electrons are always shown using dots.
Multiple Bonds
The bonds on the previous department are called unmarried bonds. Each bail contains two electrons (one bonding pair). A pair of atoms can also share four electrons or six electrons. If the atoms share four electrons, the bond is called a double bond. For example, the bail in O2 is a double bond.

A double bond is usually depicted with two parallel lines.
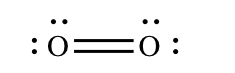
If the atoms share 6 electrons, the bail is called a triple bail. Triple bonds are rather rare, but the bail in Northward2 is a triple bail.
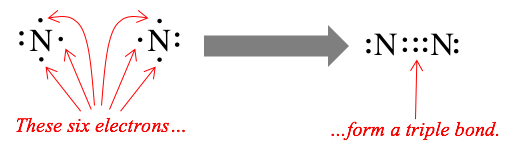
A triple bond is depicted with three parallel lines.

Drawing Lewis structures for molecules with one central cantlet: ii rules
In Chem 101A, we will focus on cartoon Lewis structures of molecules and polyatomic ions that have i cardinal cantlet with several other atoms attached to it. Such molecules are very common, and they provide a foundation for understanding structures of more complex molecules. To begin, you must know two essential rules for drawing Lewis structures.
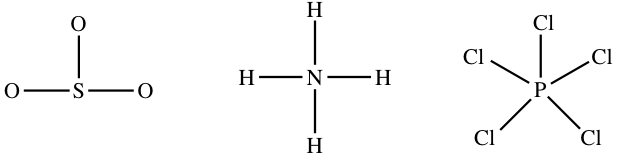
Rule 1: In any molecule or ion with the full general formula ABn , the unique atom (A) is in the center and all of the B atoms are attached to A.
For case, the basic arrangements of the atoms in And theniii, NH4 +, and PCl5 are:
Exercise not e'er describe a structure like the ones below!
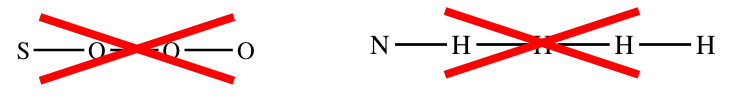
Rule 2: Lewis structures are not intended to show the actual shape of the molecule; they just prove which atoms are bonded to each other.
None of the molecules in a higher place actually await like the structures here. For example, the bodily shape of So3 is:
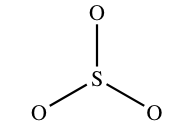
Drawing Lewis structures for molecules with ane central atom: five steps to success
The post-obit procedure will give you the correct Lewis structure for whatsoever molecule or polyatomic ion that has one central atom.
Step 1: Figure out how many electrons the molecule must take, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to business relationship for the charge.
Pace two: Connect the atoms to each other with unmarried bonds to form a "skeleton construction." Exist sure that you follow rule one in the previous department.
Step 3: Add plenty electrons (dots) to the outer atoms to requite each of them a full of eight electrons around them. (Exception: do not add together electrons to hydrogen atoms.) This tendency of atoms to have 8 electrons around them is chosen the octet rule.
Step iv: Count the electrons in your structure. If you demand to add together whatsoever more than based on your count in step ane, add together them to the central atom.The electron count in your final reply must match the count from step 1.
Step v:
- If the central cantlet has eight or more electrons around it, you're finished.
- If the fundamental atom has fewer than 8 electrons around it, simply all of the surrounding atoms are from group 7A, you're finished.
- Otherwise, move a nonbonding electron pair from an outer atom to a bail (i.due east. make a double bond). If the central cantlet now has viii electrons around it, you're finished. Otherwise, repeat this procedure until the central atom has 8 electrons.
Example: drawing the Lewis structure of CO3 ii –
Step 1) Figure out how many electrons the molecule must accept.
Carbon has 4 valence electrons
Each oxygen has half dozen valence electrons
The -2 charge means that there are two actress electrons
Total: 4 + (3 × half-dozen) + 2 = 24 electrons
The final answer MUST have this number of electrons‼!
Step 2) Attach the atoms to each other using single bonds ("draw the skeleton structure")
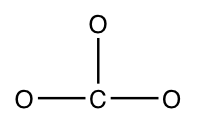
Step 3) Add electrons to all outer atoms (except H) to consummate their octets.
The outer atoms are the oxygen atoms hither. Each outer atom needs three electron pairs, since information technology already has one bonding pair.
(1 line = 2 electrons)
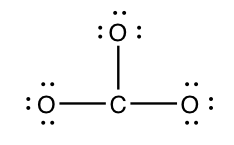
Step 4) Count the electrons in the structure.
This structure has 24 electrons.
three lines = 6 bonding electrons
xviii dots = eighteen nonbonding electrons
total = 24 electrons
Does this match the count y'all got in pace 1?
COthree ii –: should have 24 electrons (from step 1)
Our structure has 24 electrons – check! It MATCHES!
Step 5)
If the fundamental cantlet has 8 or more than electrons, yous're done.
The carbon atom has but half-dozen electrons effectually information technology, so we aren't finished yet…
If the central atom has fewer than 8 electrons, but all of the outer atoms are in group 7A, y'all're done.
The outer atoms are oxygen atoms, and oxygen is in group 6A, so we aren't finished withal…
Otherwise, create an extra bond by changing one of the nonbonding pairs into a bonding pair. (Exception: don't do this if all of the outer atoms are from group 7A.)
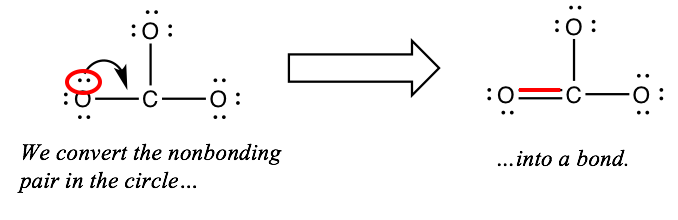
Discover that this stride does non change the full number of electrons in our structure. It just moves ii electrons to a dissimilar location.
If the central atom nonetheless has fewer than 8 electrons around information technology, practise this every bit many more times as y'all need. But do not become across 8 electrons on the key atom. When you reach 8 electrons, you're done.
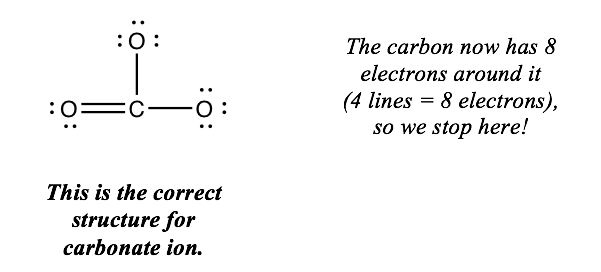
Chemists ofttimes draw foursquare brackets effectually the construction of a polyatomic ion and write the charge outside the brackets, like this:
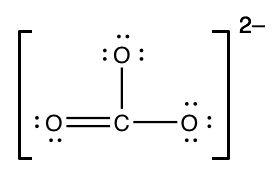
Notation: nosotros could have put the double bail in two other locations. Any of the three options is fine; you only need to draw one of them.
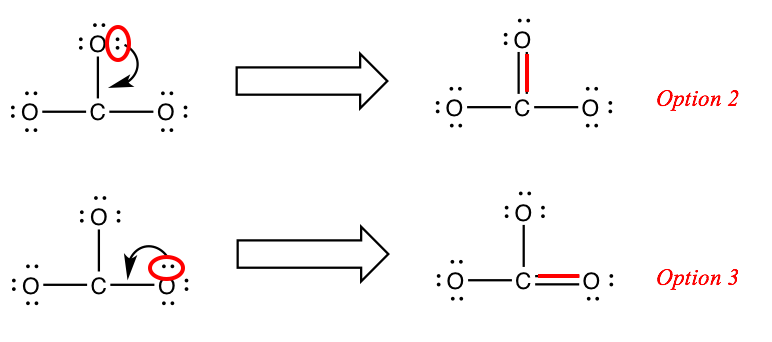
(If you're wondering "what almost resonance??"… we'll get to that subsequently.)
Instance: Drawing Lewis structures for BFthree, PF3 and BrFiii
Next, we'll look at three molecules side-by-side. The molecules are BF3, PF3, and BrFthree, all of which take a fundamental atom bonded to iii fluorine atoms.
Step 1) Figure out how many electrons each molecule must have.
BFthree: 3 + 7 + 7 + 7 = 24 electrons
PFiii: 5 + 7 + 7 + vii = 26 electrons
BrFthree: vii + 7 + 7 + vii = 28 electrons
The concluding answers MUST take these numbers of electrons‼!
Stride 2) Attach the atoms to each other using unmarried bonds ("draw the skeleton structure").

Step 3) Add electrons to the outer atoms, to complete their octets.
Each outer atom needs three electron pairs.
Stride 4) Count the electrons in each construction.
Each of these structures has 24 electrons.
3 lines = 6 bonding electrons
18 dots = 18 nonbonding electron
total = 24 electrons
Exercise these match the counts you got in footstep i?
BFthree: should accept 24 electrons (from stride 1)
Our construction has 24 electrons – MATCHES
PF3: should have 26 electrons (from footstep 1)
Our structure has 24 electrons
Nosotros NEED TO Add together 2 MORE ELECTRONS
BrF3: should take 28 electrons (from pace ane)
Our structure has 24 electrons
WE Demand TO ADD 4 More than ELECTRONS
If the counts do not match, add the remaining electrons to the central atom.
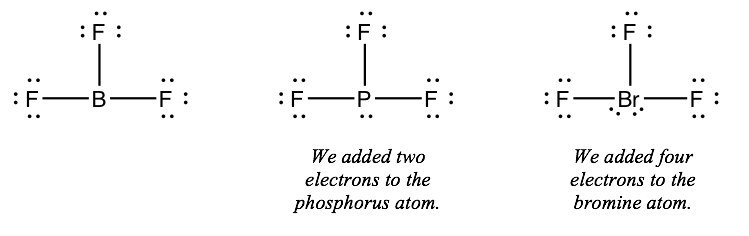
Stride 5) If the central cantlet has 8 or more electrons around information technology, you're done.
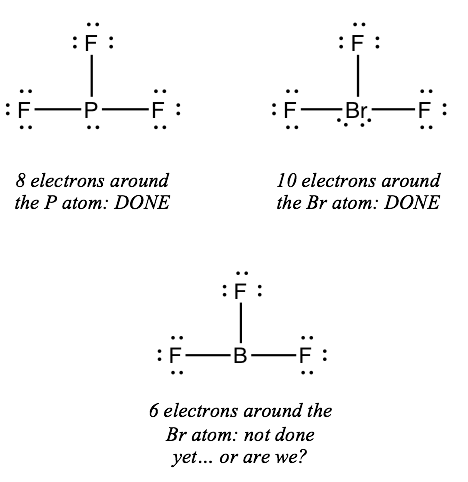
If the key atom has fewer than 8 electrons around it, just all of the outer atoms are in group 7A, you're washed
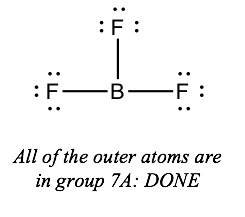
Note: these half-dozen elements from group 7A are chosen halogens: F, Cl, Br, I At, Tn. You'll merely see the first 4 of them in chemical compounds; the last two are extremely radioactive.
Breaking the Octet Rule
The central atom in a construction often violates the octet rule. Electron-scarce atoms are rare, but expanded octets are fairly common with elements in the 3rd row and beyond.
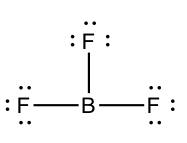
In BFiii, the central atom simply has 6 electrons around it. We say that the boron atom is electron deficient.
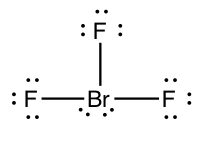
In BrFiii, the primal atom has 10 electrons around information technology. We say that the bromine cantlet has an expanded octet.
Notation that information technology is too quite common for the key atom to make more than iv bonds. Here are a couple of examples:
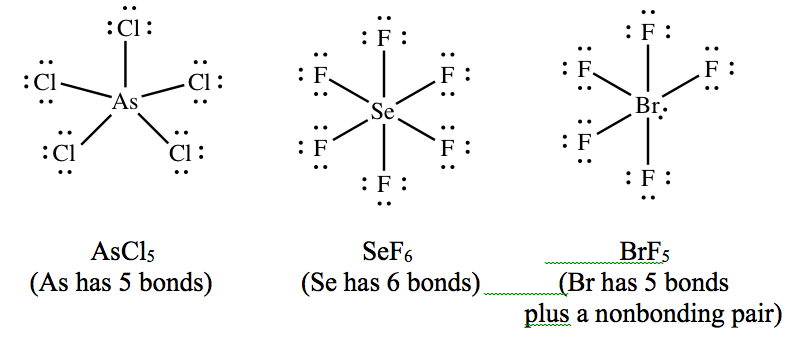
Don't panic if you see such molecules. Just follow the rules for drawing Lewis structures, and you lot'll get them right!Yet, you must note that outer atoms never violate the octet rule.
Using formal charges to determine how many bonds to make, a dissimilar perspective...
If we depict the Lewis construction for PO4 3 – ion using the rules yous've seen earlier, nosotros come upwards with the construction beneath. It has the correct number of electrons (32), and every atom satisfies the octet rule.
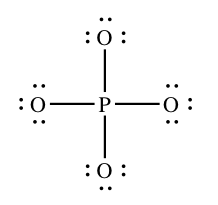
However, many textbooks (and websites) insist that the structure below is a amend ane, even though the phosphorus cantlet has ten electrons effectually it:
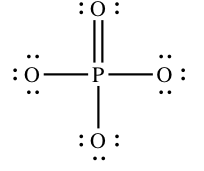
The beginning structure follows the rules for drawing structures. So why practice chemists add together a double bail? To understand, we need the formal charges on the atoms in each structure…
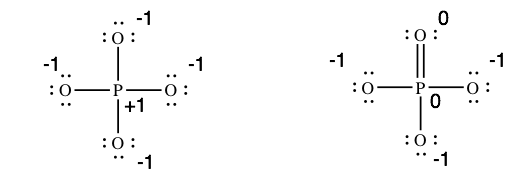
Many chemists prefer the second structure considering it gives u.s.a. a couple of zeroes. Nevertheless, the phosphorus atom in the 2nd structure violates the octet rule. So which structure is best?? The answer is that either structure is legitimate. Both structures give usa all of the information nosotros need about phosphate ion; they allow us to predict the shape of the molecule, the angles between the bonds, and whether the molecule is polar.
So which construction should Y'all draw on a test? Be sure to check with your instructor, simply most will accept either one. Even so, the first structure is easier to figure out, considering it's the construction you produce when you follow the provided rules. The second construction requires more piece of work. Therefore, we recommend that when you lot draw a structure that satisfies the octet dominion, you lot terminate there without adding more bonds.
Alert: Some students come to Chem 101A having been taught to draw Lewis structures with extra double bonds.
If you're familiar with Lewis structures and you like the extra bonds… congratulations! You have less to larn – you already know all of this stuff.
Merely…
If you lot draw structures with extra bonds, they accept to be correct. For example, a structure for phosphate that has two double bonds is not adequate.
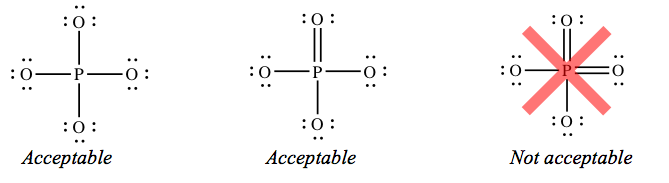
As well, a structure for nitrate ion (NO3 –) that has two double bonds is non acceptable, fifty-fifty though it gives you lot more zeroes. The construction with 1 double bond is the simply acceptable 1.
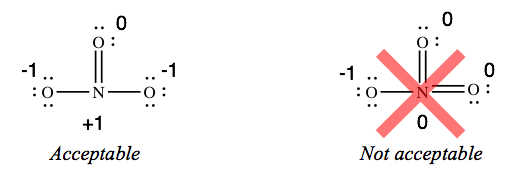
If you don't know why the structures I've labeled "unacceptable" are not immune, don't risk losing points past adding extra bonds when the key cantlet already has eight electrons.
Using formal charges to evaluate which is the best central cantlet...
Mostly, yous are told which cantlet is the central bonding atom in the molecule. However, if it is unclear, or if you are asked to decide which atom is central, formal charges tin be used to decide. The following is an example.
Example: calculating the formal charges in HCN and HNC
For the arrangement HCN, the Lewis structure: H–C\(\equiv\)N:
The formal charges piece of work out as follows:
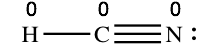
For the arrangement HNC, the Lewis structure: H–N\(\equiv\)C:
The formal charges work out equally follows:
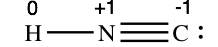
Both Lewis structures have a net formal charge of nil, only note that the formal charges on the outset structure are all zip! Thus the first Lewis structure is predicted to exist more stable, and it is, in fact, the construction observed experimentally. In general, the closer the formal charges are to nil, the more stable the structure. Call back, though, that formal charges do not represent the actual charges on atoms in a molecule or ion. They are used but as a book-keeping method for predicting the most stable Lewis structure for a compound.
Contributors and Attributions
James Armstrong, City Higher of San Francisco
Torrey Glenn, City College of San Francisco
Source: https://chem.libretexts.org/Courses/City_College_of_San_Francisco/Chemistry_101A/Topic_F%3A_Molecular_Structure/09%3A_Basic_Concepts_of_Covalent_Bonding/9.03%3A_Drawing_Lewis_Structures
Posted by: aquinowassent.blogspot.com


0 Response to "How To Draw Lewis Structures .doc .pdf"
Post a Comment